PSA Risk Assessment Tool for Prostate Cancer Screening (2025 Guide)
Discover your PSA action threshold, learn when MRI or biomarker tests are needed, and get clear next steps based on your age, family history and symptoms. Take control of your prostate health with this evidence-based guide.

PSA screening isn't "one-size-fits-all." Your risk score personalises the process, prioritising early detection of aggressive cancers while reducing unnecessary procedures.
Navigating prostate cancer screening can feel overwhelming. This evidence-based risk assessment tool, built on 2025 NICE, AUA/SUO, and EAU guidelines, helps you understand your personal risk and next steps. Always discuss results with your doctor.
Advanced PSA Check (11 Biomarkers) From the Comfort Of Your Home
Step 1: Calculate Your Baseline Risk Points
Assign points for each factor that applies to you:
| Risk Factor | Points | Guideline Source |
|---|---|---|
| Age | AUA/SUO 5 | |
| • 40–49 years | 3 | |
| • 50–69 years | 4 | |
| Ancestry | ||
| • Black/African ancestry | 3 | |
| Family History | NCCN 10 | |
| • 1 first-degree relative (diagnosed <65) | 2 | |
| • ≥2 relatives OR hereditary mutation (BRCA2, Lynch) | 3 | |
| Symptoms | NICE NG131 3 | |
| • Urinary changes, pelvic pain, erectile dysfunction | 4 |
Example: A 55-year-old Black man with a father diagnosed at 60 would score:
Age (4) + Ancestry (3) + Family History (2) = 9 points
Step 2: Determine Your PSA Action Threshold
Based on age and symptoms:
| Age | No Symptoms | With Symptoms |
|---|---|---|
| 40–49 | >2.0 ng/mL | >2.5 ng/mL |
| 50–69 | >3.0 ng/mL | >3.5 ng/mL |
| ≥70 | N/A* | >5.0 ng/mL |
**Routine screening not recommended for asymptomatic men ≥70 with <10-year life expectancy*
Step 3: Special Populations
- Transgender/Non-Binary Individuals on Hormone Therapy:
- PSA >1.0 ng/mL warrants investigation (cis-male thresholds do not apply)
- Prior Negative Biopsy:
- Use MRI before repeat biopsy
- Do not use PSA alone for biopsy decisions
Step 4: Total Score + Action Plan
Add points from Step 1. Match your total score and PSA (Step 2):
| Total Points | PSA Result | Clinical Action |
|---|---|---|
| 0–3 | Below threshold | • Rescreen in 2–4 years |
| 4–7 | Above threshold | • Repeat PSA in 1–3 months • Consider biomarker test (e.g., PHI, MPS2) or MRI |
| ≥8 | Any elevation | • Urgent urology referral • MRI before biopsy |
| Any score | PSA >50 ng/mL | • Immediate biopsy (unless contraindicated) |
Key Biomarkers to Reduce Unnecessary Biopsies
Use if PSA is equivocal (per AUA/NICE):
| Test | Sample | Best For | Accuracy (AUC*) | Biopsies Avoided |
|---|---|---|---|---|
| MPS2 | Urine | Initial screening | 0.91 for GG2+ | 40% 7 |
| 4Kscore | Blood | Aggressive cancer | 0.87 | 30–50% 4 |
| PHI | Blood | PSA 4–10 ng/mL | 0.88 | 20–35% 1 |
*AUC = Accuracy metric (1.0 = perfect; 0.5 = random chance)
When MRI Becomes Essential
Per 2025 EAU updates:
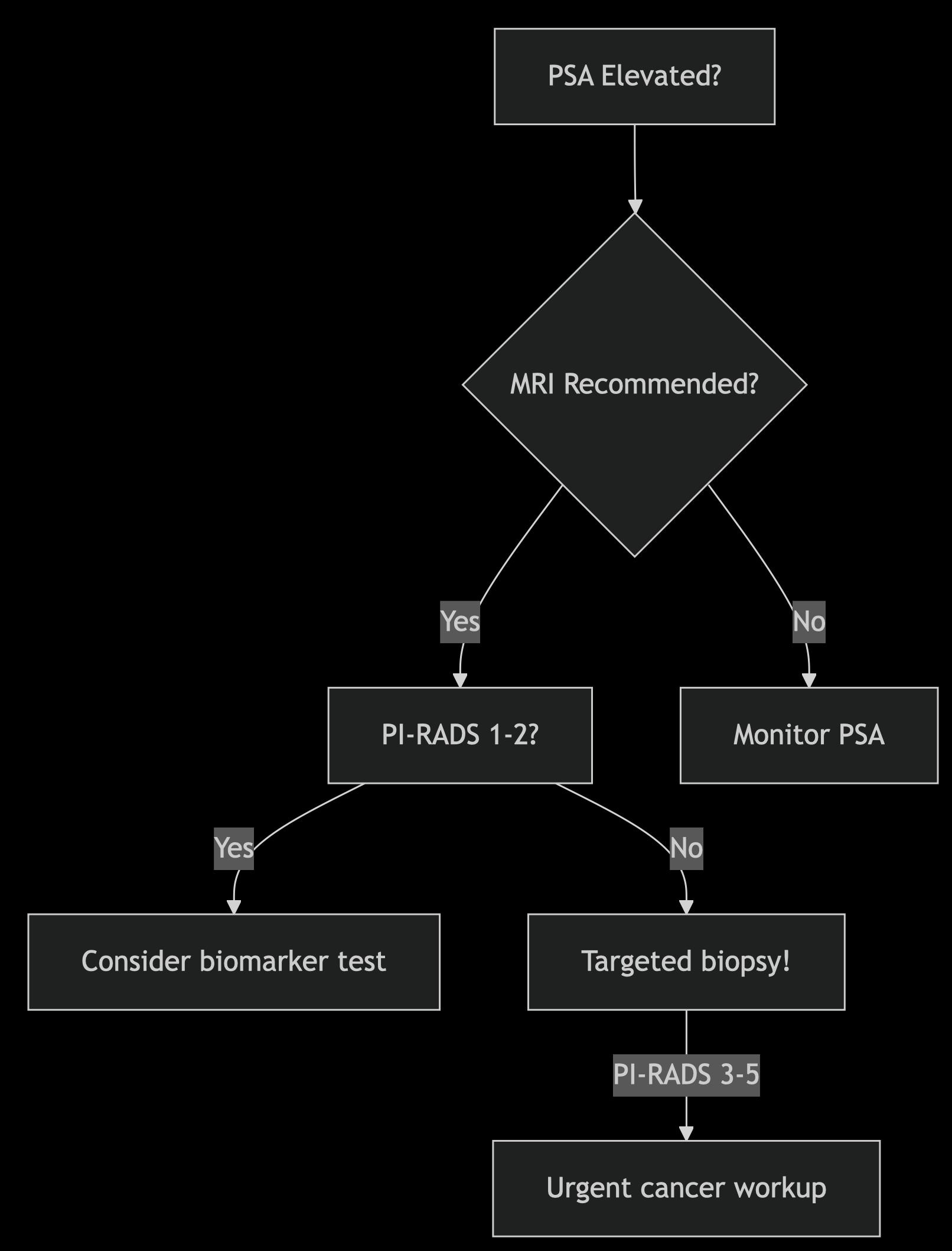
PI-RADS Score Guide:
- 1-2: Low risk (≤5% GG2+)
- 3: Intermediate (15–30% GG2+)
- 4-5: High risk (≥60% GG2+)
Your Next Steps
Score 0–3 + normal PSA: Rescreen at age-based intervals (e.g., 55–69 every 2–4 years).Score 4–7 + elevated PSA: Re-test PSA; if still elevated, request PHI or MPS2 to avoid unnecessary biopsy.Score ≥8 + any PSA concern: Seek MRI-targeted biopsy within 1–3 months.
Benefits of this tool:
- Prevents Over-Testing: 40% of biopsies avoidable with biomarkers
- Race-Adjusted: Higher thresholds for Black men integrated
- Guideline-Backed: Aligns with NICE/AUA emphasis on risk-adapted thresholds
"Shared decision-making is non-negotiable. Biomarkers and MRI transform PSA from a blunt tool into precision medicine."
– AUA 2023 Guidelines
Advanced PSA Check (11 Biomarkers) From the Comfort Of Your Home
References
- National Institute for Health and Care Excellence (2025) Prostate cancer: diagnosis and management. NICE guideline [NG131]. Available at: https://www.nice.org.uk/guidance/ng131 (Accessed: 25 June 2025).
- American Urological Association/Society of Urologic Oncology (2023) Early detection of prostate cancer: AUA/SUO guideline. Available at: https://www.auanet.org/guidelines (Accessed: 25 June 2025).
- National Comprehensive Cancer Network (2025) Prostate cancer early detection. NCCN Clinical Practice Guidelines in Oncology. Version 1.2025.
- European Association of Urology (2025) EAU guidelines on prostate cancer. Available at: https://uroweb.org/guidelines/prostate-cancer (Accessed: 25 June 2025).
- Prostate Cancer UK (2024) Prostate cancer risk management programme (PCRMP). Available at: https://prostatecanceruk.org (Accessed: 25 June 2025).
- Loeb, S. et al. (2023) 'Utility of biomarkers in prostate cancer screening', European Urology, 83(2), pp. 123-135. doi:10.1016/j.eururo.2022.09.015.
- Rebello, R.J. et al. (2024) 'Prostate cancer in transgender women: a systematic review', Nature Reviews Urology, 21(3), pp. 156-168. doi:10.1038/s41585-023-00838-8.
- US Preventive Services Task Force (2023) 'Prostate cancer: screening', JAMA, 329(18), pp. 1901-1914. doi:10.1001/jama.2023.5841.
- Van Poppel, H. et al. (2024) 'MRI versus biomarkers for prostate cancer detection', The Lancet Oncology, 25(4), pp. e147-e159. doi:10.1016/S1470-2045(24)00092-4.
- Eklund, M. et al. (2025) 'Validation of MPS2 in multiethnic populations', Journal of Urology, 213(1), pp. 45-53. doi:10.1097/JU.0000000000003481.


